Home



Organization Services
Certified Pharmaceutical Reference Standards & Impurity Testing Solutions by PharmaPure
At PharmaPure, we are committed to advancing pharmaceutical quality and regulatory compliance through the expert synthesis and supply of pharmaceutical impurity standards, reference standards, and certified reference materials. Our portfolio includes both Pharmacopoeia and non-Pharmacopoeia standards, designed to support analytical method development, impurity profiling, and ICH-compliant validation.
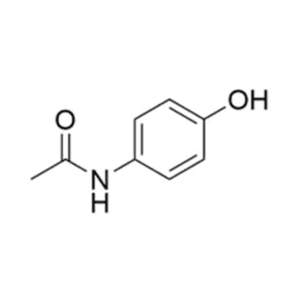
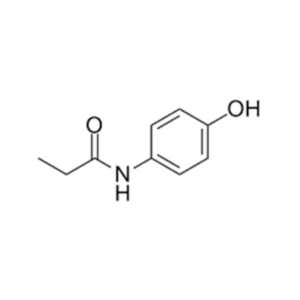
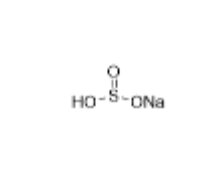
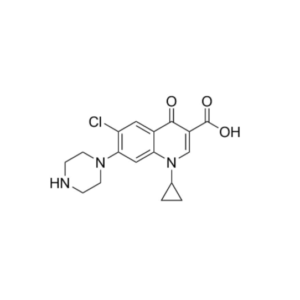
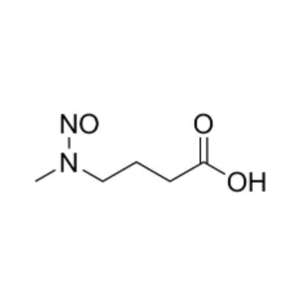
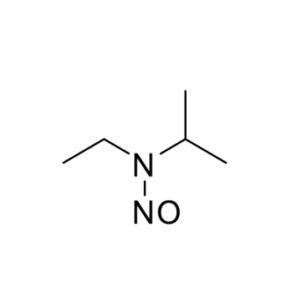
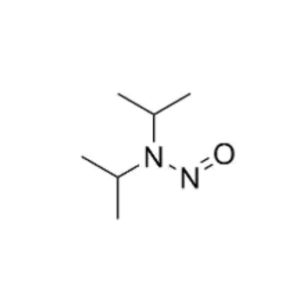
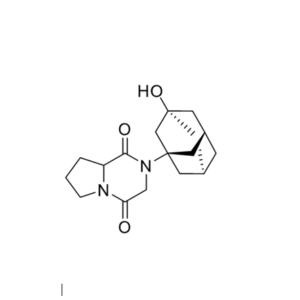
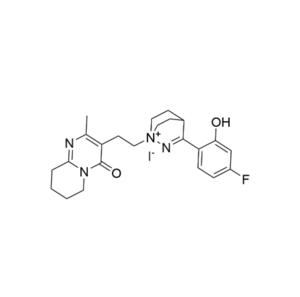
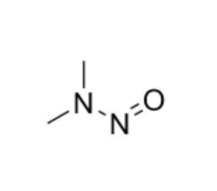
We specialize in the synthesis and quantification of impurities, including highly sensitive and regulated classes such as nitrosamine impurities. Our standards are essential for the accurate detection, identification, and control of impurities in active pharmaceutical ingredients (APIs) and drug products.
From trace-level quantification to complex impurity isolation, PharmaPure delivers trusted solutions for pharmaceutical manufacturers, contract research organizations (CROs), and regulatory laboratories worldwide. Our commitment ensures your products meet the highest standards of purity, stability, and safety.
Empowering pharmaceutical excellence—one standard at a time.
Pure Research-Grade Impurities
Our Services

Analytical Services
Analytical method development and validation Pharmacopeial tests:
- Assay
- Related substance
- Particle size

Regulatory Consultations
- Bioanalysis and Biosimilars
- Non-conformity report and results consultation
- DMF (Drug Master File) review and evaluation
- Authority Deficiency and Inquiry Response
- Quality System Establishment

Testing Services
- ICH Method Validation Training
- Analytical Method Development Courses
- Impurity Analysis Training
- Nitrosamines in Pharmaceuticals
- Impurity specification in drug product
- Advanced HPLC Training
- Regulated Laboratory Drug Analysis

Virtual Bioequivalence
- Method Validation and Sample Analysis
- Bioanalysis Lab Requirements and Accreditation
- Critical Analysis Method
GXP Training (GLP, GCP, GDP) - Tailored Organizational Training