Home



Organization Services
Your Trusted Source for High-Quality Lab Chemicals
Pharmapure is a premier provider of pharmaceutical reference standards, specializing in both Active Pharmaceutical Ingredients and impurities. Our expert team, deeply rooted in the pharmaceutical industry, is dedicated to supporting you with exceptional after-sales services.
We pride ourselves on delivering high-quality standards that meet the stringent requirements of the industry, ensuring your research and production processes are efficient and reliable. At Pharmapure, we are committed to innovation, quality, and customer satisfaction, making us your trusted partner in pharmaceutical excellence.
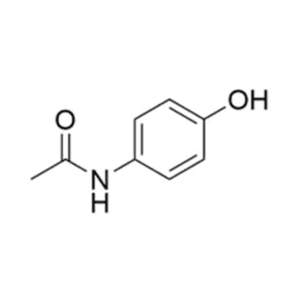
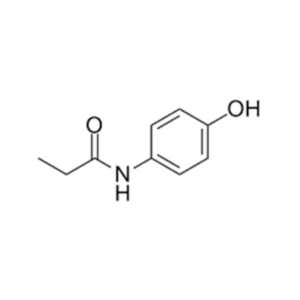
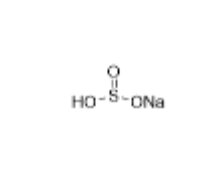
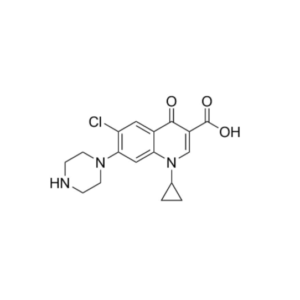
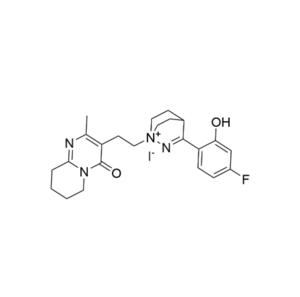
Pure Research-Grade Impurities
Our Services

Pharma R&D Services
Pharmaceutical Formulation Development
Analytical method development and validation Pharmacopeial tests:
Analytical method development and validation Pharmacopeial tests:
- Assay
- Related substance
- Particle size

Consultation
- Bioanalysis and Biosimilars
- Non-conformity report and results consultation
- DMF (Drug Master File) review and evaluation
- Authority Deficiency and Inquiry Response
- Quality System Establishment

Pharma Training
- ICH Method Validation Training
- Analytical Method Development Courses
- Impurity Analysis Training
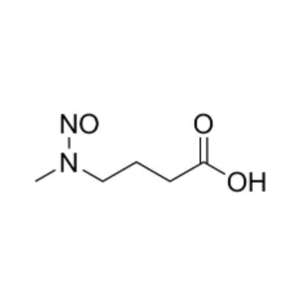
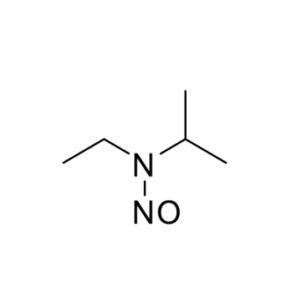
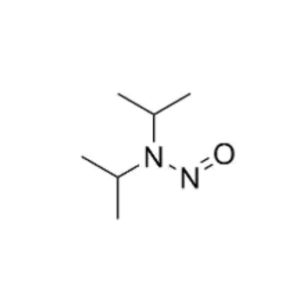
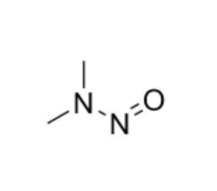
- Nitrosamines in Pharmaceuticals
- Impurity specification in drug product
- Advanced HPLC Training
- Regulated Laboratory Drug Analysis

Bioanalysis Training
- Method Validation and Sample Analysis
- Bioanalysis Lab Requirements and Accreditation
- Critical Analysis Method
GXP Training (GLP, GCP, GDP) - Tailored Organizational Training
- Method of analysis development and validation
- ICH Stability studies
- Dissolution profiles
- Stress studies (forced degradation)
- Nitrosamines Testing by LC-MS/MS and GC-MS/MS
- Metals testing by ICPMS (ICH Q3D)
- Cleaning validation
- QC testing of batches
- Leachable and Extractables for packaging materials
- Cross-contamination studies
Pharma R&D services
Pharma Training
Bioanalysis Training
Quality Consultation
Pharma R&D Consultation
Bioanalysis Consultation
Biosimilar Consultation